- Caring for the Vine - January 23, 2026
- The Global Ayahuasca Boom: What About the Conservation of the Ayahuasca Vine? - July 15, 2022
- Cactus Conservation Institute Clarification Note about Decriminalize Nature Claims - June 14, 2022
Tabernanthe iboga, or bois sacré as it is known in French, is a perennial shrub found in the undergrowth of the tropical forests of the Congo Basin. This plant can be found in several West African countries, but because of increase in demand for iboga, escalating deforestation, and complex political and economic situations in the countries where it grows, there are concerns for its sustainability.

The Bwiti also have weekly ceremonies called ngoze, or “masses,” in which members of the community take much smaller doses of powdered iboga in connection with ritual and ceremonial dances.
T. iboga can be considered a cultural keystone species, meaning that it is of exceptional significance to a culture or a people (Quiroz & Andel, 2018). It is integral for the Bwiti religion, practiced in Gabon and, to a lesser extent, in other Central African countries. The rootbark of this shrub is rasped and eaten raw or made into a powder or drink by the Bwiti practitioners. Sometimes it is mixed with honey or other medicinal plants. Large doses of iboga are taken during initiation rites to connect with the ancestors for guidance. The Bwiti also have weekly ceremonies called ngoze, or “masses,” in which members of the community take much smaller doses of powdered iboga in connection with ritual and ceremonial dances. In small doses, iboga is also traditionally used as stimulant and to counter fatigue and increase performance (Fernandez & Fernandez, 2001).
In the Bwiti legends, the Pygmies are said to have discovered iboga by observing wild animals. Hogs could be seen digging holes at the foot of the iboga trees to get the root bark, and shortly after, they would fall into a wild frenzy. Porcupines and parrots also enjoy the roots (Pope, 1969). Reports of gorillas eating the root bark are localized to certain areas, and the use is infrequent, indicating possible use as medicinal tonic (Cousins & Huffman, 2002).
Discover the Indigenous Reciprocity Initiative of the Americas
However, unlike classic psychedelics, ibogaine is more dangerous, and higher doses or pre-existing vulnerabilities, such as cardiovascular problems, can lead to overdoses, paralysis and death
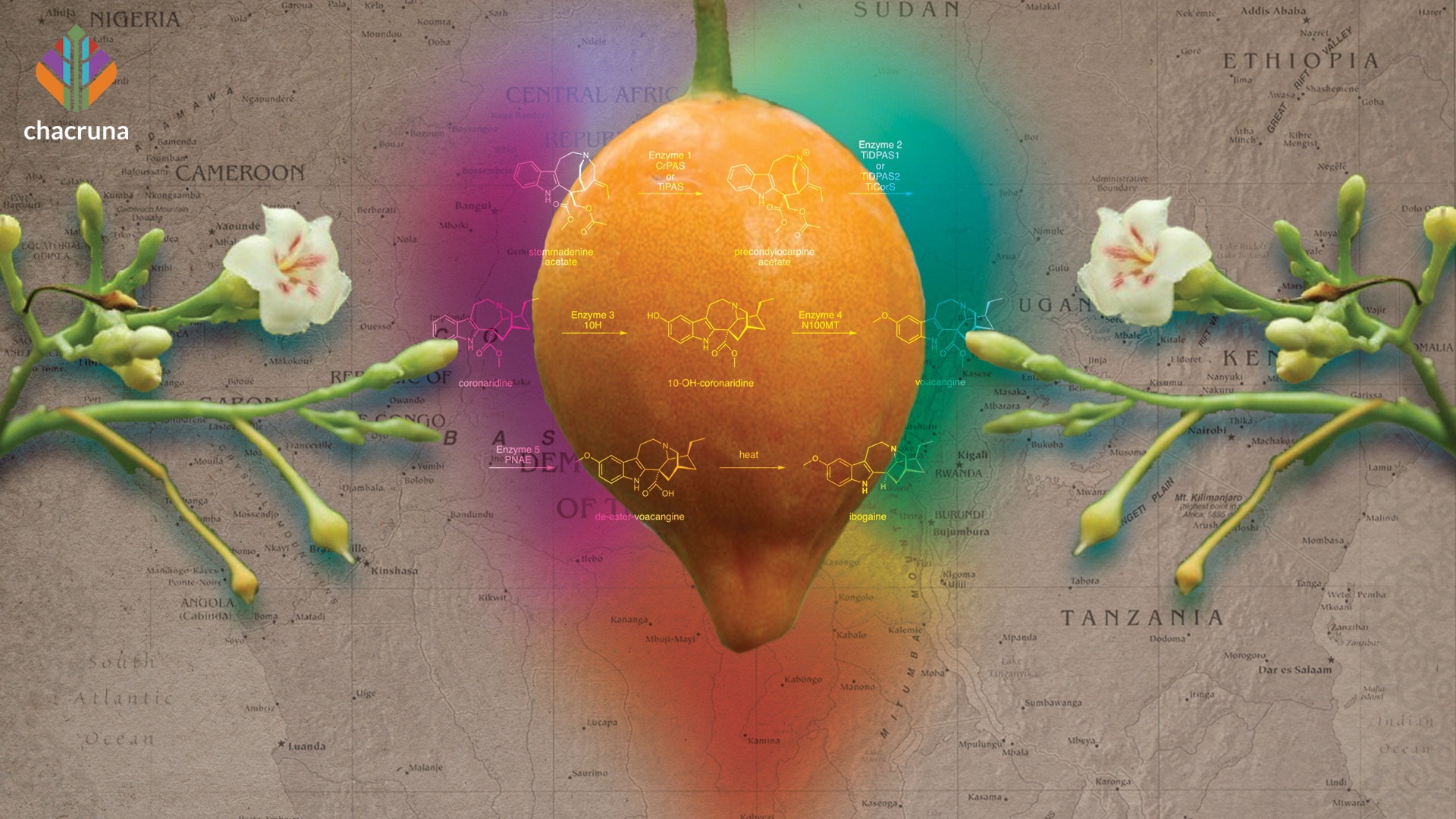
T. iboga has several bioactive alkaloids, but the principal psychoactive among them is ibogaine. Ibogaine was used in Western medicine since the early 1900s, and was even popular as stimulant for athletic performance, until its ban in the 1960s (Gallo et al., 2009). Howard Lotsof championed iboga since 1962 as an addiction treatment. Ibogaine therapy centers are appearing around the globe, and several clinical trials using ibogaine or similar compounds, are underway to assess its effectiveness. Preliminary preclinical and clinical studies demonstrate promising results in the treatment of opioid and other addictions (Wasko et al., 2018). However, unlike classic psychedelics, ibogaine is more dangerous, and higher doses or pre-existing vulnerabilities, such as cardiovascular problems, can lead to overdoses, paralysis and death (Litjens & Brunt, 2016).
Harvesting Practices
The rootbark is the most valued and used part of the plant, as it contains the highest concentration of ibogaine. How much ibogaine there is in the root bark depends on many factors: plant age, soil, symbiotic relationship with other trees; or, possibly, many other factors. Ibogaine level in the root bark is highly variable according to the literature, ranging from 0.27%–10% (Iyer et al., 2020; Bouso et al., 2020). The tree takes 7–10 years to mature. For harvesting, the tree must be at least five years old, although the older, the better. A five-year-old iboga plant can produce about 250 grams of rootbark, and a 20 to 30-year-old plant can produce 1 kg or even 2kg of rootbark.
It is possible to harvest the rootbark of the iboga tree without killing it. This method consists of exposing the roots of the plant by digging the soil around it, peeling the bark off some of the roots, and then replacing the soil cover. When harvested with this method, the tree survives, although it grows slower. The period of rotation, where the tree is left to recover, should be at least 3 years. However, some people completely uproot the tree to harvest all the bark (Tonye, 2000). If this is the case, a good practice is to replant a few cuttings from the uprooted plant, to ensure future propagation.
Conservation Status
The IUCN Red List of Threatened Species lists T. iboga as of “least concern,” citing a wide distribution across several West African countries and allegedly large populations (IUCN, 2018). However, there are no detailed biological studies or assessments available in English, nor other languages in Gabon or elsewhere it is endemic to, i.e., Cameroon, both Congos, Angola, Equatorial Guinea, and the Central Africa Republic. Likewise, it is not known how many species of the genus Tabernanthe there are, and which ones are distinct species and which ones are varieties.
In Gabon, iboga is often sold in the traditional pharmacies and markets of Libreville, Oyem, and Bitam. Much of the trade occurs between Bwiti members and traditional healers in Central Africa, although international export is of growing concern (Towns et al., 2014). Converging evidence from various sources indicates shortages in Gabon, particularly in urban centers like Libreville, with prices quoted increasing 10-fold in the last decade (Dickinson, 2015). The Gabonese branch of the Wildlife Conservation Society in Libreville expressed concern about the unsustainable export-oriented harvest of Tabernanthe iboga, but no official data on exported species or volumes were available (Towns et al., 2014). As of 2019, Gabon’s Ministry of Agriculture made it illegal to export iboga, unless the plant has been cultivated on private land and all permits are obtained from the Ministry of Forestry and the Environment.
In 2000, the Council of Ministers of the Republic of Gabon declared Tabernanthe iboga to be a national treasure, or “cultural heritage strategic reserve.” It is now governed by several laws, most notably the Convention on Biodiversity (Rio 1992, ratified in Gabon in 1997), Law for the Protection of Cultural Goods (December 10, 1994), and the Nagoya Protocol (Signed in Gabon on July, 2012). Yet, overharvesting and poaching still pose a threat.
There is very little information in scientific literature about distribution, species abundance, or the conservation status of T. iboga. The information below is based on the four key recent reports, all centered in Gabon. One was a report commissioned by the Gabonese government, led by Yann Guignon and Jean-Nicolas Dénarié, (2012). The other three reports, based on qualitative interviews with the international ibogaine community (283 respondents, 55 interviewed and 228 people surveyed from 12 and 34 countries, respectively) and, most importantly, on fieldwork and engagement with local stakeholders from Gabon (56 people from 12 Bwiti communities interviewed) were led by ICEERS (Faura & Langlois, Phase I, 2019; Phase II, 2020; Phase III, 2021).
Little care is given to the fact that overharvesting and export of iboga impacts the Bwiti, the traditional community who use the plant ceremonially as a part of their spiritual practice.
All three reports cite a sharp rise in global consumption of iboga rootbark, and production of ibogaine as serious concern. This leads to the increase in poaching and international shipments of iboga out of the country. Little care is given to the fact that overharvesting and export of iboga impacts the Bwiti, the traditional community who use the plant ceremonially as a part of their spiritual practice.
Locally, this results in shortage of iboga for the local communities, particularly in urban centers in Gabon, and an increase in prices. These local shortages are significant because, unlike in many other West African countries, the majority of Gabonese population is urban. This shortage is exacerbated by the Gabonese police seizing domestic shipments; what happens later to these shipments is not known. The scarcity results sometimes in “false” iboga being sold (e.g., rootbark from another plant that looks similar, like Rauvolfia monbasiana or Pterotaberna inconspicua, and that are also used in traditional medicine), which presents health risks for those ingesting it (at least one death has been reported, see Gicquel et al., 2016).
Recordings are now available to watch here
Deforestation is another alarming factor. Even though in Gabon, there is an extensive network of national parks, many of the remaining forests are under threat from logging and lumber trade. A large portion of the common forests are under logging and mining concessions (ForestWatch, 2021), and even if they are selectively logged and not razed entirely, they are fenced off, and access for the local people is restricted. Moreover, even in the protected areas, there are reports of poaching.
Iboga’s fruits attract, among other animals, elephants and gorillas who spread the plant’s seeds. As these animals dwindle due to hunting and human development, we lose not only them, but also an important step in the lifecycle of iboga.
Climate change also plays a role, whereby the rainy seasons in Gabon have become shorter and the temperature has increased, adding stress to the whole ecosystem. Iboga’s fruits attract, among other animals, elephants and gorillas who spread the plant’s seeds. As these animals dwindle due to hunting and human development, we lose not only them, but also an important step in the lifecycle of iboga. Iboga poaching is also sometimes linked to the ivory trade.
Additional factors are Christian (particularly Evangelical Christian religions) and Islamic influences that marginalize traditional Bwiti practitioners, leading to problems in generating political and financial support; for example, for cultivation projects. For this reason, some Bwiti communities welcome interest from the foreigners, as it adds to legitimacy and can potentially bring more international support for local initiatives.
Cultivation
Traditionally, iboga is collected from the wild, where it was freely available until recently. Only a small number of plants were planted around houses, graveyards, or churches in the Bwiti communities.
Although some sellers of iboga and ibogaine claim it comes from cultivated sources, organizations like Blessings of the Forest indicate that this is not the case; most of iboga, both for local and international trade, still comes from wild-harvested sources. In collaboration with the wildlife organization, Conservation Justice, Blessings of the Forest documented numerous cases of iboga being illegally harvested in Gabon and traded internationally from Cameroon by individuals, groups, and even poaching organizations who have also been implicated in the ivory and pangolin black markets.
Iboga was always freely available in the forests, and harvested there; therefore, there is a lack of traditional knowledge pertaining to cultivation. Likewise, very few scientific cultivation and propagation trials have been conducted with T. iboga in Central Africa.
Ever since it became glaringly obvious that the use of ibogaine outside of Africa was putting a great deal of strain on traditional practices within Gabon, and the poaching and wild harvesting of this precious root was affecting the supply and the ecosystem, there have been some incredible efforts to establish cultivated iboga in Gabon for Gabonese use, as well as outside of Gabon. For example, there are a few manufacturers of T. Iboga-derived medicines that are working with iboga cultivated in Ghana, the Ivory Coast, and Cameroon, and there are people planting it in other tropical forests across the world, such as Costa Rica, Mexico, Brazil, Peru, and other countries. Time will show how these trees fare, and whether they will provide a sufficient supply of suitable medicine.
What Are the Alternatives?
In addition to the genus Tabernanthe, Tabernaemontana and Voacanga are viable sources of therapeutic iboga alkaloids (they are all from Apocynaceae family). Phytochemical proximity between Tabernanthe, Tabernnaemontana, and Voacanga genera opens a lot more plants as a source of anti-addictive compounds, and will hopefully contribute to a reduction in harvesting of T. iboga in Africa (Krengel et al., 2019; Iyer et. al., 2020).
According to the ICEERS survey, among the international ibogaine community they surveyed, Tabernanthe iboga was the primary source for both rootbark (62% to 67%) and the ibogaine alkaloid (49% to 56%). Alternative sources, such as ibogaine semi-synthesized from Voacanga africana accounted for about 20%, while Tabernanthe manii or Tabernaemontana were used by about 3% of respondents.
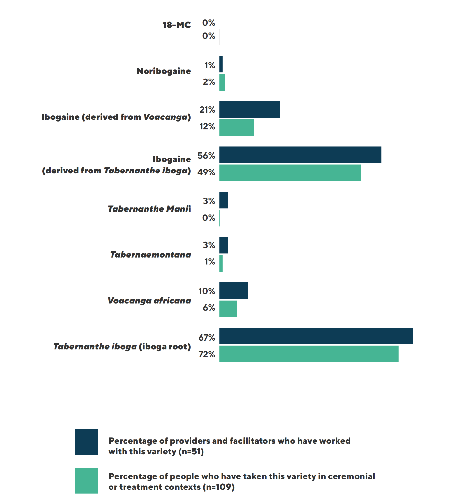
However, the market is dynamic and has changed since Voacangine-derived ibogaine was manufactured and sold about 11 years ago. It is becoming more common now for providers and clinics to use ibogaine hydrochloride semi-synthesized from Voacanga.
Not only is it more sustainable, as Voacanga is much more abundant than T. Iboga in various African countries, traction is gaining to achieve GMP status, which is crucial for research and standardized dosing structures. Of course, there are practitioners who are still using ibogaine HCL derived from T. Iboga, including PTA and TA, as well as the rootbark.
It is also possible to synthesize ibogaine without the use of any plants (see review by Iyer et al., 2020), although the synthesis method that is economically feasible to scale is yet to be developed. Another option is to use ibogaine derivatives, such as such as 18-MC, which is currently undergoing several clinical trials.

Conclusion
Writing this article made me realize that there are many more questions than answers when we are considering sustainability and conservation of T. iboga. The most obvious knowledge gaps are the current absence of biological field studies, problems estimating occurrence of T. iboga across its distribution range, and a lack of understanding of the different iboga varieties. We need research into factors influencing the presence of alkaloids in the bark, and different ways of cultivating and growing iboga. Moreover, where cultivation is concerned, it is necessary to develop cultivation methods that are least invasive and, ideally, do not involve monoculture plantations. It would be good to support and promote cultivation programs where iboga is grown in the forest, such as in the community-managed protected areas. It is necessary to establish a tracing system to be able to know where T. iboga is coming from, and whether it was grown and harvested sustainably.
Another question concerns the international ibogaine community: How much ibogaine does the world need? What are the most ethical ways of satisfying this demand without exacerbating availability and prices locally? How can this be approached with respect and reciprocity to the Bwiti community? What does biocultural sustainability look like for iboga?
Blandine Akendengue is a professor at the University of Health Sciences in Libreville, Gabon. Here she talks about iboga sustainability and benefit sharing.
It is necessary to actively explore alternative plant sources of ibogaine and also to improve on the existing chemical synthesis, making it scalable, as well as to develop innovative technologies, such as biosynthesis of iboga by yeast, as is already possible for psilocybin.
It is likely that even increasing cultivation of T. iboga will not sufficiently satisfy the growing demand. It is necessary to actively explore alternative plant sources of ibogaine and also to improve on the existing chemical synthesis, making it scalable, as well as to develop innovative technologies, such as biosynthesis of iboga by yeast, as is already possible for psilocybin.
All of the above, of course, would require appropriate regulations, so that the process is transparent, balancing the interests of the diverse stakeholders involved. And, most importantly, we need to think systemically; to change our way of thinking from problem solving to an ecosystem approach. We need to embody the principle of reciprocity, of not only taking (whether it is healing, plants, or knowledge), but giving back (not only financially, although this helps) to the Pygmy and Bantu communities who hold traditional knowledge about using this plant, and to the natural environment: the forests where iboga grows.
Acknowledgements: Thank you R. Faura, H. Wells and C. Wilkins for sharing your knowledge about iboga and ibogaine.
Featured art by Trey Brasher.
References
Bouso, J. C., Fornís, I., Vilamala, M. V., Loenen, B. D., Sainz-Cort, A., Jimenez-Garrido, D. F., Santos, R. G. dos, Hallack, J. E. C., Alcázar-Córcoles, M. A., & Jenks, C. W. (2020). An analytical study of iboga alkaloids contained in Tabernanthe iboga-derived products offered by ibogaine treatment providers. Archives of Clinical Psychiatry (São Paulo), 47(2), 51–54.
Cousins, D., & Huffman, M. A. (2002). Medicinal properties in the diet of gorillas: An ethno-pharmacological evaluation. African study monographs, 23(2), 65–89.
Fernandez, J. W., & Fernandez, R. L. (2001). “Returning to the path”: The use of iboga [ine] in an equatorial African ritual context and the binding of time, space, and social relationships. The Alkaloids. Chemistry and biology, 56, 235–247. https://doi.org/10.1016/s0099-9598(01)56017-4
Gallo, C., Renzi, P., Loizzo, S., Loizzo, A., & Capasso, A. (2009). Tabernanthe iboga: A comprehensive review. Pharmacologyonline, 3, 906–920.
Gicquel, T., Hugbart, C., Le Devehat, F., Lepage, S., Baert, A., Bouvet, R., & Morel, I. (2016). Death related to consumption of Rauvolfia sp. powder mislabelled as Tabernanthe iboga. Forensic Science International, 266, e38–e42.
Krengel, F., Chevalier, Q., Dickinson, J., Herrera Santoyo, J., & Reyes Chilpa, R. (2019). Metabolite profiling of anti‐addictive alkaloids from four Mexican Tabernaemontana species and the entheogenic African shrub Tabernanthe iboga (Apocynaceae). Chemistry & Biodiversity, 16(4), e1800506.
Litjens, R. P., & Brunt, T. M. (2016). How toxic is ibogaine? Clinical Toxicology, 54(4), 297–302.
Pope, H. G. (1969). Tabernanthe iboga: An African narcotic plant of social importance. Economic Botany, 23(2), 174–184.
Quiroz, D., & van Andel, T. (2018). The cultural importance of plants in Western African religions. Economic botany, 72(3), 251–262.
Tonye-Mahop, M., Asaha, S., Ndam, N., Blackmore, P. (2000). State of knowledge study on Tabernanthe iboga baillon: A report for the Central African regional program for the environment. Washington, DC: United States Agency for International Development (USAID),.
Towns, A. M., Quiroz, D., Guinee, L., de Boer, H., & van Andel, T. (2014). Volume, value and floristic diversity of Gabon’s medicinal plant markets. Journal of Ethnopharmacology, 155(2), 1184–1193.
Take a minute to browse our stock:
Did you enjoy reading this article?
Please support Chacruna's work by donating to us. We are an independent organization and we offer free education and advocacy for psychedelic plant medicines. We are a team of dedicated volunteers!
Can you help Chacruna advance cultural understanding around these substances?