The first report that ibogaine could reduce opioid withdrawal symptoms and opioid craving dates back to the early 1960s.1 Since then, there has been growing enthusiasm for its use in treating a range of substance use disorders. Although a drug with antiaddiction properties could be of immense importance for addiction and mental health services, most of ibogaine’s clinical use has occurred outside of mainstream medical practice.2 Perhaps as a reflection of this, the scientific literature on ibogaine’s activity in humans is sparse, consisting mainly of uncontrolled case studies.3 For a drug with apparently promising clinical activity, how has this situation arisen? Is there a pathway for ibogaine or its analogues to get regulatory approval for clinical use? I have approached these questions from the perspective of conventional pharmaceutical development.
Randomized controlled trials (RCTs) are central to convincing physicians and regulatory agencies that a drug is safe and effective. There are no RCT data showing the effects of ibogaine on relevant endpoints (e.g., drug craving, drug use). In order to set up ibogaine RCTs, we need clearer information on many as-yet-unresolved questions, including:
- How should ibogaine be dosed (how much, how frequently, how to manage relapses, etc.). Although a range of ibogaine doses and dosing regimens have been reported, these appear to be pragmatic rather than being based on any sort of formal assessment.
- Which addictive drugs should be evaluated? Clinical case series and animal addiction models appear to show ibogaine has activity against multiple drugs of dependence. However no pharmaceutical has yet been approved for treatment of multiple substance use disorders, and it would be difficult to design an RCT which included patients with, for example, alcohol, cocaine or opioid use disorders. Including multiple substances in a single trial would be difficult because of because of different substance-specific withdrawal symptoms, different durations of withdrawal, etc.
Besides having to demonstrate that ibogaine is effective, ibogaine’s safety needs to be characterized to the extent that appropriate risk:benefit assessments can be made. Several groups have reported that ibogaine and noribogaine block activity at a type of potassium channel involved with heart repolarization, and thus have the potential to cause heart rhythm disturbances or arrhythmias.4 5 I recently completed research with single doses of noribogaine in opioid-dependent patients wanting to come off methadone.6 This study showed a clear association between higher noribogaine concentrations in blood and electrocardiogram changes associated with an increased risk of heart arrhythmias. In this situation, a risk:benefit assessment would evaluate the risk of harm or death from an arrhythmia against the benefit of preventing death or complications arising from continued use of substances.
Because the risk of arrhythmia is associated with ibogaine and noribogaine concentrations in blood, dose is another important modifiable factor in this assessment. For example, a dosing regimen that gave multiple low doses of noribogaine over the course of several days would achieve lower peak concentrations than a single large dose (see Figure 1), and may be safer clinically because of lower risk for heart arrhythmias.
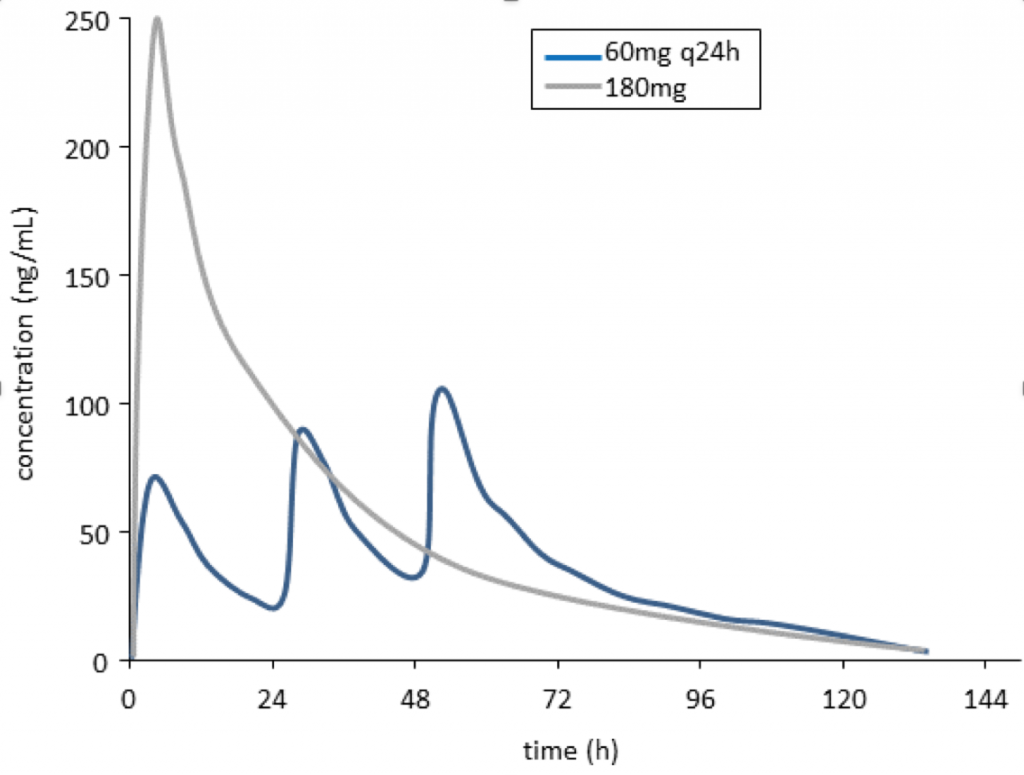
Figure 1: Simulated noribogaine concentration-time profiles after a single 180mg dose (grey line) compared with 3 x 60mg doses given at 24 h intervals (blue line). Drug exposure (area under the concentration-time curve) is the same for both dosage regimens; however, peak noribogaine concentrations are lower for the split dose regimen.
Another important safety consideration relates to variability in how ibogaine is converted to noribogaine in the body by an enzyme called CYP2D6. The activity of this enzyme is reduced or absent in 5-10% of Caucasians. In such patients, ibogaine can be converted to noribogaine by different enzymes; however, the process is much less efficient, resulting in much higher ibogaine blood concentrations for up to 48 hours post-dose. I recently completed a study where we converted healthy volunteers into CYP2D6 poor metabolizers by dosing them with a CYP2D6 inhibitor, paroxetine. These subjects had 2-fold higher exposures to ibogaine and noribogaine compared with volunteers who had normal CYP2D6 activity.7 It will be important to check CYP2D6 activity before dosing patients with ibogaine so that lower doses can be given to CYP2D6 poor metabolizers.
Drug manufacturing is a third important area of scrutiny for regulatory agencies. This involves technical issues such as purity of drug substance, how the drug is formulated, batch size, and how stable the drug is during storage, among other factors. These are evaluated according to a set of agreed international standards.8 There are laboratories where the ibogaine drug is manufactured according to Good Manufacturing Practice (GMP) standards; however, relevant formulation and stability studies are unlikely to exist.
Because of the cost and complexity of clinical trials and the registration process, a sponsor is essential. For commercial sponsors (Big Pharma), the incentive to run clinical studies is based on recouping research costs and getting income from product sales after a drug is approved by drug regulatory agencies. It is essential to have some form of patent protection to protect sales from cheaper generic (unbranded) drugs. The lack of interest by commercial sponsors to develop ibogaine has been reported,9 and is probably linked to difficulties obtaining patent protection, along with concerns about the size of the market for medications to treat substance use disorders. At present, two small companies, DemeRx and Savant HWP, have sponsored clinical trials, with ibogaine’s active metabolite noribogaine and an ibogaine analog, 18-MC, respectively. For either drug to achieve regulatory approval, the financial and logistical resources of a larger pharmaceutical company would likely be needed to run large clinical trials and manage registration. There are parallels between ibogaine’s nonstandard development journey and that of lithium, a mood stabilizer. John Cade, an Australian psychiatrist, described lithium’s activity in mania in 1949.10 However, it was only approved by the US FDA for this indication 21 years later, in 1970. (In contrast, the average time now for clinical testing and FDA approval for central nervous system drugs is 7.6 years11). A major factor in lithium’s sluggish regulatory approval was the absence of a commercial sponsor.
There are alternatives to commercial sponsors, such as the non-profit research organization Multidisciplinary Association for Psychedelic Studies (MAPS). An example of MAPS’ non-commercial approach is their coordination and sponsorship of studies of MDMA-guided psychotherapy for Post-Traumatic Stress Disorder.12 More importantly, they have proposed a series of clinical and regulatory steps to use these data, and future research, to achieve FDA approval for MDMA for this indication. MAPS is co-ordinating observational research of ibogaine treatment in Mexico and New Zealand; however, no plans have been reported for developing clinical trials or seeking regulatory approval.
For ibogaine or analogues to reach their full therapeutic potential in treating addictions, they need to be approved for clinical use by drug regulatory agencies in major countries. This has not yet happened, due to the absence of efficacy and safety data. The analogues noribogaine and 18-MC have ongoing small development programs, and may have the potential to achieve registration if larger pharma sponsors become involved. MAPS may offer an alternative, non-commercial pathway to registration for ibogaine. Characterizing and managing cardiovascular safety will be essential in any of these programs.
Disclosure: I am a psychiatrist who spent two decades in clinical research in the pharmaceutical industry in the UK and USA.
References
- Alper K, Beal, D., & Kaplan, C. D. (2001). A contemporary history of ibogaine in the United States and Europe. In K. Alper & G. A. Cordell (Eds.), The alkaloids: Chemistry and biology, Vol. 56, (pp. 249-281). London: Academic Press/Elsevier. ↩
- Alper K., Lotsoff, H. S., & Kaplan, C. D. (2008). The ibogaine medical subculture. J Ethnopharmacol., 115(1), 9–24. ↩
- Dos Santos R. G., Bouso, J. C., & Hallak, J. E. (2017). The antiaddictive effects of ibogaine: A systematic literature review of human studies. J Psychedelic Stud, (0),19 DOI: 10.1556/2054.01.2016.001 ↩
- Alper, K., Bai, R., Liu, N., Fowler, S. J., Huang, X., Priori, S. G., & Ruan, Y. (2016). hERG Blockade by iboga alkaloids. Cardiovasc Toxicol 16(1),14–22. ↩
- Koenig, X., & Hilber K. (2015). The anti-addiction drug ibogaine and the heart: A delicate relation. Molecules, 20(2), 2208–2228. ↩
- Glue, P., Cape, G., Tunnicliff, D., Lockhart, M. Lam, F., Hung, N. … Friedhoff, L. (2016).. Ascending single-dose, double-blind, placebo-controlled safety study of noribogaine in opioid-dependent patients. Clin Pharmacol Drug Devel., 5(6), 460–468. ↩
- Glue, P., Winter, H., Garbe, K., Jakobi, H., Lyudin, A., Lenagh-Glue, Z., & Hung, C. T. (2015). Influence of CYP2D6 activity on the pharmacokinetics and pharmacodynamics of a single 20 mg dose of ibogaine in healthy volunteers. J Clin Pharmacol., 55(6), 680–687. ↩
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). http://www.ich.org/home.html ↩
- Alper K. (2001). Ibogaine: A review. In K. Alper & G. A. Cordell (Eds.), The alkaloids: Chemistry and biology, Vol. 56 (pp. 1–38). London: Academic Press/Elsevier. ↩
- horter E. (2009). The history of lithium therapy. Bipolar Dis., 11(0),4-9. ↩
- Kaitin, K. I., & DiMasi, J. A.. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000-2009. Clin Pharmacol Ther., 89(2), 183 –188. ↩
- MAPS. http://www.maps.org/about ↩
Take a minute to browse our stock:
Did you enjoy reading this article?
Please support Chacruna's work by donating to us. We are an independent organization and we offer free education and advocacy for psychedelic plant medicines. We are a team of dedicated volunteers!
Can you help Chacruna advance cultural understanding around these substances?