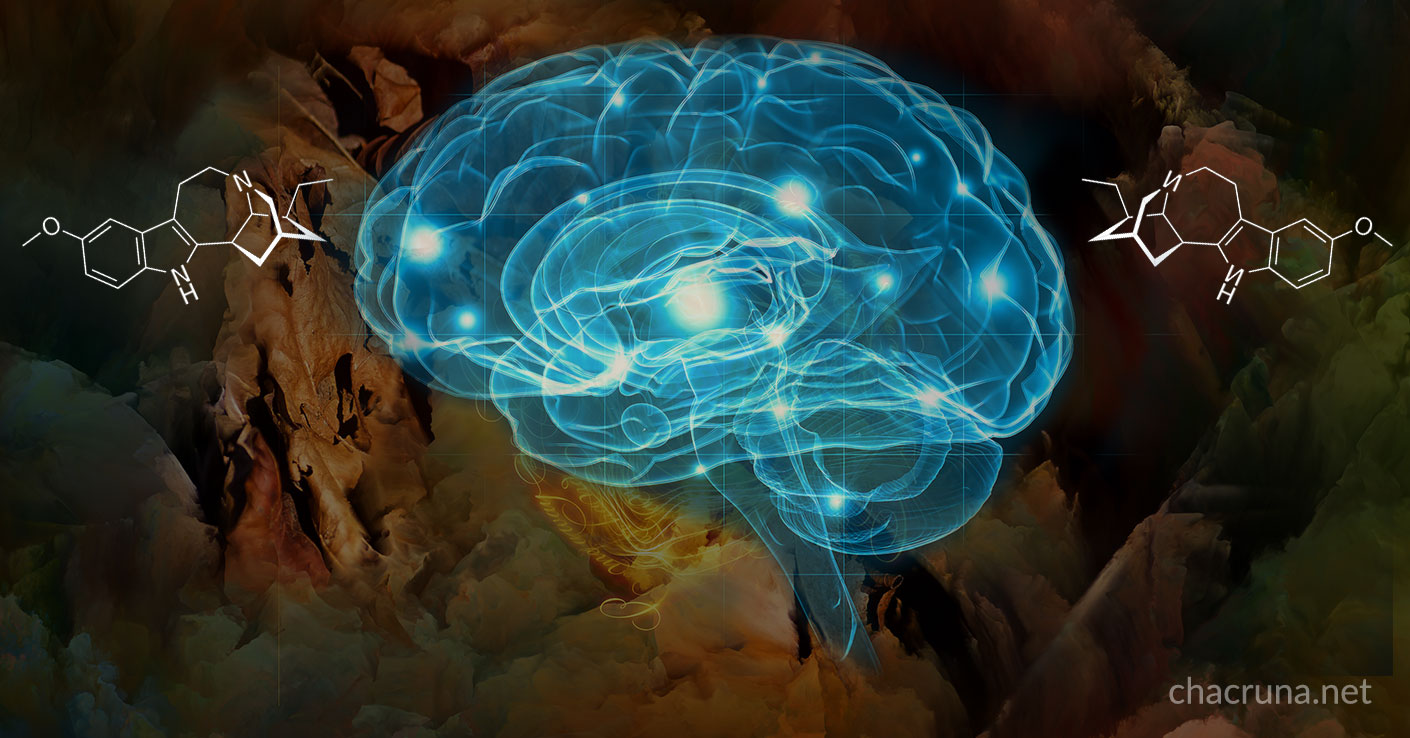
- How the Psychedelic Ibogaine May Heal, Repair & Protect the Brain - April 13, 2017
Ibogaine is a potent psychedelic substance found mainly in the root bark of the shrub Tabernanthe iboga, native of the Congo and Gabon, as well as in other plants of the Apocynaceae family. It has been used in rituals for spiritual growth and medical purposes in the Bwiti spiritual tradition. In the Western world, ibogaine has become widely known because of its beneficial effects on drug addiction.1 These effects have been confirmed in studies on rodents, where ibogaine decreases seeking behavior for morphine, heroin, cocaine, nicotine, and alcohol.2 3 4 5 6 For humans, the reduction of drug craving by ibogaine have been highlighted in many anecdotal reports.
Rigorous studies on humans to test this substance as a medication for treating addiction have not been completed, and some potential negative side effects have been found for ibogaine. For instance, it has been shown to have a delicate relationship with the heart, with the potential risk of producing fatal arrhythmias in subjects with preexisting cardiovascular problems or other medical conditions.7 As a chemist, understanding the mechanism of action of ibogaine is essential to developing new substances that could retain the anti-addictive proprieties without having undesired side effects.
What does ibogaine do in the brain to promote these anti-addictive effects? Using a computer as a metaphor, ibogaine has effects on both the “hardware” (e.g., neural circuitry, and neurotransmitters and their receptors) and “software” (e.g., personality, ego structure) of the brain. Effects on the “software” are very potent because of the psychedelic nature of the drug, and a lot of users have claimed that the insight gained by these effects has allowed them to pursue profound changes in their lives, including changing their relationship with their drugs of abuse. This potential has also been deeply explored in psychotherapeutic settings.8 9 10
Regarding the “hardware”, ibogaine has effects on several receptors in the brain and in the neurotransmitter systems. Especially important for our ongoing research is that ibogaine can promote the release of small proteins called “neurotrophic factors” in some parts of the brain.11 12
What are these neurotrophic factors? These are substances that promote survival, repair and protection processes in the brain tissue.13 In addition, these substances can promote neurogenesis (the generation of new neurons from progenitor cells) in the developing nervous system and in some areas of the mature human brain.14 These substances are of vital importance for the development and function of the nervous system. It is known from studies in animals that a healthy lifestyle, physical exercise, and living in a beneficial environment produce high amounts of some of these survival signals in the brain.15 In the opposite direction, a stressful lifestyle,16 along with different brain diseases such as Parkinson’s or Alzheimer’s disease,17 and psychiatric disorders such as depression,18 alter normal neurogenesis and the content of neurotrophic factors in several brain areas. Since ibogaine can promote the release of these neurotrophic factors in some regions of the brain, it is proposed it could repair and protect the damaged neural circuits involved in drug addiction.19 20
With these results as a background, we are studying the ability of ibogaine to alter neurotrophic factors production in different brain areas, using rodents as an animal model, especially in brain regions that are involved in drug abuse. We want to understand the mechanism by which ibogaine produces the release of these small proteins to identify potential pharmacological targets.
We are also preparing new molecules in the lab, which share a similar chemical structure with ibogaine, and testing them in brain cells, to assay their ability to increase the production of certain neurotrophic factors. In this way, being inspired by the ibogaine´s molecular structure, we want to discover the relationship between the chemical structure of our compounds and their ability to release neurotrophic factors in the brain. Through our research, we believe we may soon be able to identify potent and safer substances that could be used for the treatment of drug abuse and other pathologies of the central nervous system.
This work is being developed in the Universidad de la República (Facultad de Química – Facultad de Medicina) and the Instituto de Investigaciones Biológicas Clemente Estable, Montevideo-Uruguay in collaboration with Prof. Patricia Cassina, Prof. Gustavo Seoane and Prof. Cecilia Scorza, and with the graduate students Sebastián Rodríguez, José Pedro Prieto, Mariana Pazos, Paola Rodríguez and Bruno Gonzalez.
References
- Alper, K. (2001) Ibogaine: A review. The Alkaloids, 56, 1–38 ↩
- Glick, S., & Maisonneuve, I. M. (2000). Development of novel medications for drug addiction: The legacy of an African shrub. Annals of the New York Academy of Sciences, 909, 88–103 ↩
- Glick, S. Rossman, K. L., Steinforf, S., & Carlson, J. N. (1991). Effects and aftereffects of ibogaine on morphine self-administration in rats. European Journal of Pharmacology, 195(3), 341–345 ↩
- Cappendijk, S. L. & Dzoljic, M. R. (1993). Inhibitory effects of ibogaine on cocaine self-administration in rats. European Journal of Pharmacology, 241, 261265 ↩
- Glick, S. D., Kuehne, M. E,, Raucci, J., Wilson, T. E, Larson, D., Keller, R, W. Jr., & Carlson, J. N. (1994). Effects of iboga alkaloids on morphine and cocaine self-administration in rats: relationship to tremorigenic effects and to effects on dopamine release in nucleus accumbens and striatum. Brain Research, 657(1–2), 14–22 ↩
- Rezvani, A. H., Overstreet, D. H., & Lee, Y. W. (1995). Attenuation of alcohol intake by ibogaine in three strains of alcohol-preferring rats. Pharmacology, Biochemistry, and Behavior, 52(3), 615–620 ↩
- Alper, K. R., Stajić, M., & Gill, J. R. (2012). Fatalities temporally associated with the ingestion of ibogaine. Journal of Forensic Sciences, 57(2), 398–412 ↩
- Naranjo, C. (1969). Psychotherapeutic possibilities of new fantasy-enhancing drugs. Clinical Toxicology, 2, 209–224 ↩
- Naranjo, C. (1973). The healing journey. New York City, NY: Random House. ↩
- Naranjo, C. (2016). Exploraciones Psicodélicas para la transformación colectiva de la conciencia Psychedelic explorations for the collective transformation of consciousness, Barcelona: Ediciones La Llave ↩
- Dao-Yao, H., McGough, N. N. H., Ravindranathan, A., Jeanblanc, J., Marian L. Logrip,, M. L., Phamluong, K. … Dorit Ron, D. (2005). Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. The Journal of Neuroscience, 25, 619–628 ↩
- He, D. Y. & Ron, D. (2006). Autoregulation of glial cell line-derived neurotrophic factor expression: Implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 20, 2420–2422 ↩
- Levin, G., & Carter, B. D. (2014). Neurotrophic factors. New York City, NY: Springer ↩
- Zhao, C., Deng, W. & Gage, F. H. (2014). Mechanisms and functional implications of adult neurogenesis. Cell, 132(4), 645–660 ↩
- Saavedra, A., Baltazar G, & Duarte, E. P. (2008). Driving GDNF expression: The green and red traffic lights. Progress in Neurobiology, 86(3), 186–215 ↩
- Egeland, M., Zunszain, P. A., & Pariante, C. M. (2015). Molecular mechanisms in the regulation of adult neurogenesis during stress. Nature Reviews: Neuroscience, 16, 189–200 ↩
- Winner, B., & Winkler, J. (2015). Adult neurogenesis in neurodegenerative diseases. Cold Spring Harbor Perspectives in Biology, 7(4), a021287 ↩
- Duman, R. S. & Nanxin, L. (2012). A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philosophical Transactions of Royal Society of London B Bio Sci, 367(1601), 2475–2484 ↩
- Dao-Yao, H., McGough, N. N. H., Ravindranathan, A., Jeanblanc, J., Marian L. Logrip,, M. L., Phamluong, K. … Dorit Ron, D. (2005). Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. The Journal of Neuroscience, 25, 619–628 ↩
- He, D. Y. & Ron, D. (2006). Autoregulation of glial cell line-derived neurotrophic factor expression: Implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 20, 2420–2422 ↩
Take a minute to browse our stock:
Did you enjoy reading this article?
Please support Chacruna's work by donating to us. We are an independent organization and we offer free education and advocacy for psychedelic plant medicines. We are a team of dedicated volunteers!
Can you help Chacruna advance cultural understanding around these substances?
Become a Chacruna Member
To make a direct donation click the button below:
Wednesday, June 9th, 2021 from 12-1:30pm PST
REGISTER FOR THIS EVENT HERE
There is growing enthusiasm in Jewish communities about possible ancient use and modern applications of plant medicine in Jewish spiritual development. Psychedelic Judaism introduce new potential modes of healing...