Erinn Baldeschwiler, a 49-year-old mother of two teenagers, was diagnosed with stage 4 metastatic cancer and given two years to live. With tears streaming down her face, she shared in a virtual interview with The Bawmann Group, “My son’s birthday was yesterday; I just wonder how many more I’ll be able to enjoy with him” (Bawmann Group, 2021).
Erinn and Michal Bloom, patients of the Advanced Integrative Medical Science (AIMS) Institute, a Seattle-based palliative care clinic, are setting their sights on psilocybin therapy for relief from severe mental and emotional distress.
Erinn and Michal Bloom, patients of the Advanced Integrative Medical Science (AIMS) Institute, a Seattle-based palliative care clinic, are setting their sights on psilocybin therapy for relief from severe mental and emotional distress. “I have personally struggled with depression, anxiety, anger,” Erinn told Seattle Times. “This therapy is designed to really dive in and release these negative fears and shadows” (Aleccia, 2020).
Discover the Indigenous Reciprocity Initiative of the Americas
Dr. Sunil Aggarwal, Co-Founder and Co-Director of AIMS, partnered with Kathryn Tucker, a well-known patient rights attorney with Emerge Law Group. Together, they sought the U.S. Drug Enforcement Administration’s approval to provide psilocybin therapy to Erinn and Michal under federal and Washington State Right to Try (RTT) laws. Under these laws, both Erinn and Michal qualify as eligible patients with specific rights to use investigational drugs that are still in clinical trials and have yet to receive FDA approval. Psilocybin, which has passed Phase 1 clinical trials and is entering Phase 2 trials, meets the criteria for eligible investigational drugs under the RTT.
This pushed AIMS to file the nation’s first-ever lawsuit against the DEA under the premise that terminally ill patients’ rights to try are being denied, particularly because the DEA has the power to make exceptions to the Controlled Substance Act (CSA) for public health reasons. If the case is won, it has potential to establish legal precedent that could open access to psilocybin therapy for such patients nationwide.
However, even with robust research demonstrating psilocybin’s remarkable ability to alleviate treatment-resistant depression and other mental health conditions, the DEA denied Dr. Aggarwal’s request because of psilocybin’s classification as a Schedule I drug. This pushed AIMS to file the nation’s first-ever lawsuit against the DEA under the premise that terminally ill patients’ rights to try are being denied, particularly because the DEA has the power to make exceptions to the Controlled Substance Act (CSA) for public health reasons. If the case is won, it has potential to establish legal precedent that could open access to psilocybin therapy for such patients nationwide.
Right to Try Laws
The Right to Try Act was signed into federal law on May 30, 2018, and has been enacted by 41 states. RTT gives patients with life-threatening diseases a way to access investigational drugs as they do not have the luxury of time to await FDA approvals.
The Right to Try Act was signed into federal law on May 30, 2018, and has been enacted by 41 states. RTT gives patients with life-threatening diseases a way to access investigational drugs as they do not have the luxury of time to await FDA approvals. RTT can be evoked if the patient and treatment meet certain outlined criteria (Food & Drug Association, 2020).
An eligible patient is a patient who has:
- Been diagnosed with a life-threatening disease or condition;
- exhausted approved treatment options and is unable to participate in a clinical trial involving the eligible investigational drug;
- and has provided written informed consent to the treating physician
An eligible investigational drug has:
- Completed a Phase 1 clinical trial;
- not been approved or licensed by the FDA for any use;
- has an application that has been filed with the FDA or is in a clinical trial;
- has active and ongoing development or production
Kathryn, the lead attorney on the case, provided thoughts on psilocybin’s eligibility under RTT, “Can you look at the statute and see by its terms that it applies to psilocybin? I think the answer is yes” (Aleccia, 2020).
Additionally, Kathryn argues, the DEA has made exceptions to provide protection against strict federal and state laws for the purpose of public health. In one notable example, the DEA waived CSA registration requirements and permitted use of a radioactive pharmaceutical Schedule II drug. The waiver was “intended to alleviate the regulatory burdens on those administering the drug, to allow more patients to receive important diagnostic testing” (U.S. Drug Enforcement Administration [DEA], 2016). Based on legal precedent, the DEA has the ability to grant an exception to Dr. Aggarwal and acknowledge Erinn & Michal’s patient rights and eligibility under RTT.
Psilocybin Research and Proven Health Benefits
Naturally-occurring psilocybin mushrooms have been deeply respected for their medicinal qualities and included in traditional healing modalities of Indigenous tribes. Before psilocybin mushrooms were shared with Westerners, written accounts from as early as the sixteenth century document usage of psilocybin mushrooms by the Indigenous Mazatec tribe that resides in the mountains of Oaxaca, Mexico. María Sabina, an honored Mazatec curandera (healer) of her time, noted that, prior to foreign exposure, the sacred mushrooms (known as “Little Saints” or “Holy Children”), were not taken purely for spiritual experience, but were taken during healing ceremonies in order to cure the sick (Lutkajtis, 2020).
Today, psilocybin is being studied by renowned medical institutions for its ability to greatly improve treatment-resistant depression and other conditions, such as anxiety, addiction, and PTSD. Severe anxiety and depression are common among terminally-ill patients and are linked to an overall decrease in mental and emotional wellbeing and responsiveness to palliative care treatment.
Results concluded that psilocybin therapy produced “large, rapid, and sustained antidepressant effects in patients” after one or two administrations with psychological support
Clinical trials conducted by the Johns Hopkins Center for Psychedelic Research have demonstrated that psilocybin therapy is 4 times more effective than pharmaceutical antidepressants in treating participants with cancer and treatment-resistant depression. Results concluded that psilocybin therapy produced “large, rapid, and sustained antidepressant effects in patients” after one or two administrations with psychological support (Davis et al., 2020).
In another U.C.L.A study involving patients with life-threatening cancer, psilocybin “led to decreases in cancer-related demoralization and hopelessness, improved spiritual wellbeing, and increased quality of life.” (Ross et al., 2016). Improving quality of life is a fundamental objective of palliative care, making psilocybin an effective and practical option for Dr. Aggarwal to include in Erinn & Michal’s treatment.
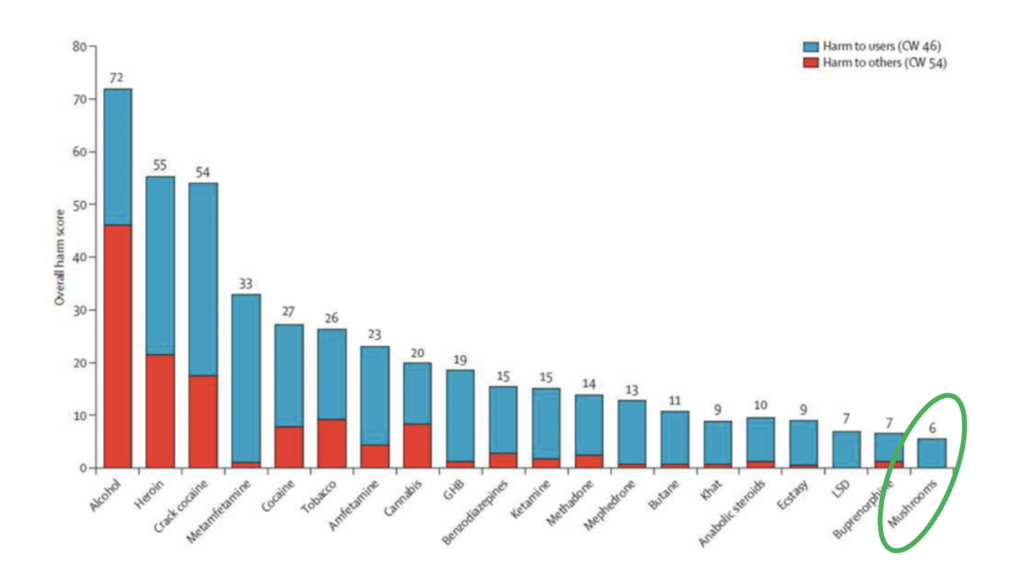
Of the 20 drugs assessed, psilocybin mushrooms were found to cause the least harm to self and to others compared to legal substances like cannabis and alcohol
Many wonder if they are safe. We turn to our friends of the Independent Scientific Committee on Drugs in the United Kingdom for a graph demonstrating the harm to self and harm to others caused by a variety of drugs. Of the 20 drugs assessed, psilocybin mushrooms were found to cause the least harm to self and to others compared to legal substances like cannabis and alcohol (see Figure 1) (Nutt et al. 2010).
AIMS et al. v. DEA
Kathryn and the Emerge Law team have been working diligently on the case against the DEA, which may have a ruling towards the end of 2021. On May 14, the legal team submitted the Petitioner’s Brief to the U.S. Ninth Circuit Court of appeals. Shortly after, the case received extraordinary support from a robust group of amici (friends of the court), including a coalition of attorney generals of eight states filing amicus briefs urging the court to rule in the patients’ favor. Although the team has made incredible progress, there is still much work to do. AIMS seeks support for this landmark case.
For more information, please see Kathryn Tucker’s blog on the Emerge Law Group website, and the RTT Advocacy Fund managed by The Nowak Society, a Colorado 501(c)(3) nonprofit.
Recordings are now available to watch here
Living and Leaving in Peace
How would you spend your final days of life? The thought seldom crosses our minds because we aren’t confronted with imminent death. However, Michal and Erinn are faced with this question. Their answers? They wish to alleviate severe anxiety and depression through psilocybin therapy, so they can better enjoy the time they have left with family and friends.
All the clinical studies, research, and quantitative data show real promise for psilocybin therapy, but its true impact is felt in the testimony of patients who have undergone treatment. Pam Sakuda, a terminally-ill participant of a U.C.L.A study, shared her experiences with psilocybin prior to her death in 2006. “I started to cry… Everything was concentrated and came welling up and then… it started to dissipate, and I started to look at it differently… I began to realize that all of this negative fear and guilt was such a hindrance… to making the most of and enjoying the healthy time that I’m having” (Slater, 2012).
In a similar Johns Hopkins study, Lauri Reamer, a survivor of adult-onset leukemia described the personal insights she gleaned from psilocybin therapy, “I now have the distinct sense that there’s so much more… so many different states of being. I have the sense that death is not the end but just part of a process, a way of moving into a different sphere, a different way of being” (Slater, 2012).
Featured art by Trey Brasher.
References
Aleccia, J. (2020, November 23). Magic mushrooms for Washington patients? New push aims to speed psilocybin to those who are dying. Seattle Times. https://www.seattletimes.com/seattle-news/health/new-legal-push-in-washington-state-aims-to-speed-magic-mushrooms-to-dying-patients/
Bawmann Group (2021, March 17). Zoom News conference with Emerge Law Group [Video file]. https://www.youtube.com/watch?v=yM6dlIev6-s
Cheers, B., Binell, M., Coleman, H., Gentle, I., Miller, G., Taylor, J., & Weetra, C. (2006). Family violence: An Australian Indigenous community tells its story. International Social Work, 49(1), 51–63. https://doi.org/10.1177/0020872806059401
Davis, A. K., Barrett, F. S., May, D. G., Cosimano, M. P., Sepeda, N. D., Johnson, M. W., Finan, P. H., & Griffiths, R. R. (2020). Effects of psilocybin-assisted therapy on major depressive disorder. JAMA Psychiatry, 78(5), 481–489. doi:10.1001/jamapsychiatry.2020.3285 https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2772630
Grob, C. S., Danforth, A. L., Chopra, G. S., Hagerty, M., McKay, C. R., Halberstadt, A. L., & Greer, G. R. (2011). Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of general psychiatry, 68(1), 71–78. https://doi.org/10.1001/archgenpsychiatry.2010.116
Lutkajtis, A. (2020). Lost saints: Desacralization, spiritual abuse and magic mushrooms. Fieldwork in Religion, 14(2) 118–139. https://journals.equinoxpub.com/OLDFIR/article/viewFile/40554/37904
https://journals.sagepub.com/doi/10.1177/0269881116675512
Nutt, D. J., King, L. A., Phillips, L. D., & Independent Scientific Committee on Drugs. (2010). Drug harms in the UK: A multicriteria decision analysis. Lancet, 376(9752), 1558–1565. https://doi.org/10.1016/S0140-6736(10)61462-6. https://pubmed.ncbi.nlm.nih.gov/21036393/
Slater, L. (2012, April 22). How psychedelic drugs can help patients face death. The New York Times. https://www.nytimes.com/2012/04/22/magazine/how-psychedelic-drugs-can-help-patients-face-death.html
U.S. Drug Enforcement Administration (2016, February 26). Removal of exemption from registration for persons authorized under U.S. nuclear regulatory commission or agreement state medical use licenses or permits and administering the drug product DaTscan. Federal Register. https://www.federalregister.gov/documents/2016/02/26/2016-04224/removal-of-exemption-from-registration-for-persons-authorized-under-us-nuclear-regulatory-commission
Take a minute to browse our stock:
Did you enjoy reading this article?
Please support Chacruna's work by donating to us. We are an independent organization and we offer free education and advocacy for psychedelic plant medicines. We are a team of dedicated volunteers!
Can you help Chacruna advance cultural understanding around these substances?



















